
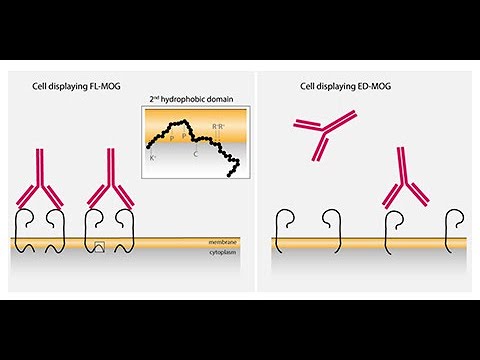
The AlexaFluor-647 median intensity is an indicator of bound human serum antibodies. The green fluorescent protein (GFP)–negative population represents nontransfected cells, and the GFP-positive population represents cells that express both acetylated GFP and myelin oligodendrocyte glycoprotein (MOG) protein. For the Mayo FACS assay, 2 cell populations are used to obtain a median fluorescent intensity. Oxford cell-based assay (CBA) (A.a) negative and (A.b) positive result Euroimmun CBA (B.a) negative and (B.b) positive result and Mayo fluorescence-activated cell sorting assay (FACS) (C.a) negative and (C.b) positive result. on behalf of the American Academy of Neurology. Both live cell-based methodologies had superior PPVs to the fixed cell assays, indicating that positive results in these assays are more reliable indicators of MOG autoimmune spectrum disorders. Overall, a high degree of agreement was observed across 3 different MOG-IgG CBAs. Of 5 false-positives, 1 was positive at both Euroimmun and Mayo and 4 were positive at Euroimmun alone. Euroimmun, Mayo, and Oxford results were as follows: clinical specificity 98.1%, 99.6%, and 100% positive predictive values (PPVs) 82.1%, 95.5%, and 100% and negative predictive values 79.0%, 78.8%, and 79.8%. Of 25 samples positive by any methodology, 21 were concordant on all 3 assays, 2 were positive at Oxford and Euroimmun, and 2 were positive only at Oxford. Seropositivity was determined by visual observation on a fluorescence microscope (Euroimmun fixed CBA, Oxford live cell CBA) or flow cytometry (Mayo live cell fluorescence-activated cell sorting assay).
The control samples were from patients with multiple sclerosis (244), hypergammaglobulinemia (42), and other (17). Serum samples from 394 patients were as follows: acute disseminated encephalomyelitis (28), seronegative neuromyelitis optica (27), optic neuritis (21 single, 2 relapsing), and longitudinally extensive (10 single, 3 recurrent). To compares 3 different myelin oligodendrocyte glycoprotein-immunoglobulin G (IgG) cell-based assays (CBAs) from 3 international centers.


 0 kommentar(er)
0 kommentar(er)
